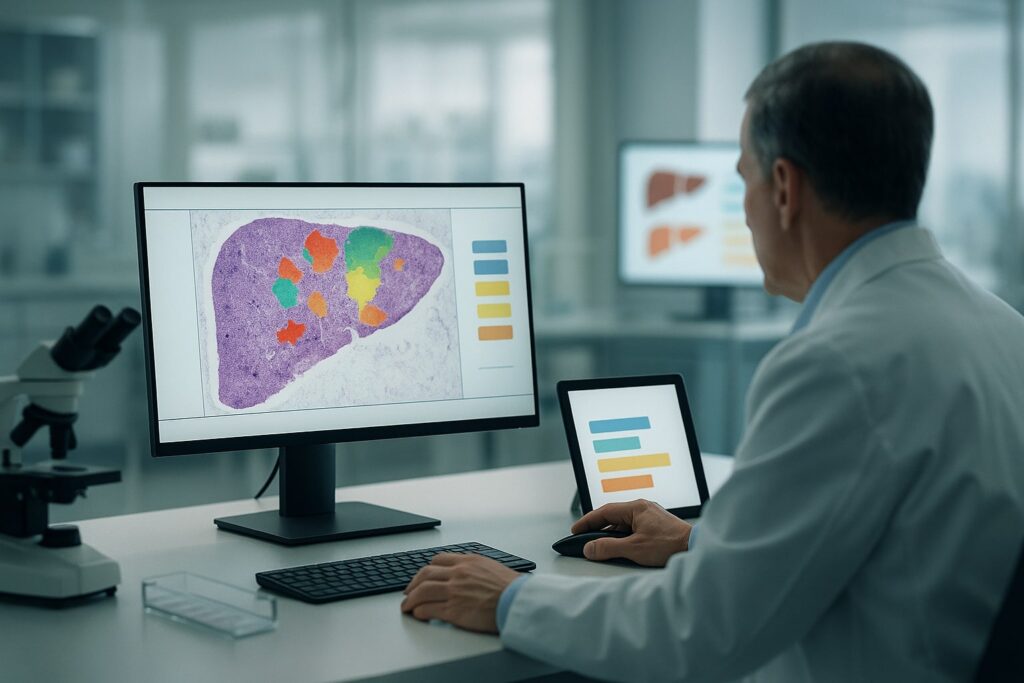
Regulators have just qualified an AI system to score liver biopsies in trials of a fast-growing liver disease. Behind the milestone lies a broader question: how far should drug development lean on algorithms that see what humans miss – and never get tired?
A quiet revolution in the liver lab
In most metabolic liver drug trials today, a patient’s fate – and often a company’s valuation – can turn on a few stained slivers of liver tissue.
Three experienced pathologists, working independently, review the same biopsy, grading fat, inflammation, ballooning of liver cells and the amount of scar tissue.